#8100 Microsporidia IFAT
Monoclonal antibodies for the
coprological diagnosis of microsporidiosis.
|
|
|
|
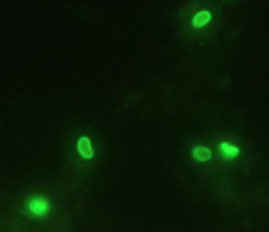
| Enterocytozoon bieneusi, mouse IgG2a FITC-conjugate: 1/60 |
Encephalitozoon intestinalis, mouse IgG1 FITC-conjugate: 1/60 |
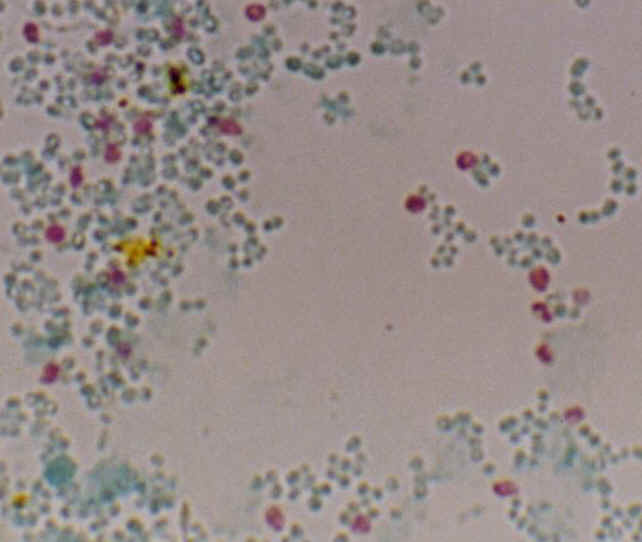
Enterocytozoon bieneusi, Trichrom stain, Weber By courtesy of M. Thellier and S. Biligui |
Enterocytozoon bieneusi and Encephalitozoon intestinalis are important opportunistic pathogens. These intracellular
protists, predominantly infect the epithelium of the small intestine causing chronic
diarrhoea and malabsorption in acquired immunodeficiency syndrome or after organ transplantation in recipients treated with powerful
immunosuppressors.
The most common microsporidial infection in humans is caused by E. bieneusi. There is a high prevalence of enteric carriage of
E. bieneusi amongst immunocompetent persons in tropical countries. This parasite naturally persists in the human population. Double infections with the two parasites might also occur.
In recent years, clinical cases caused by microsporidia decreased due to the administration of highly antiretroviral therapy (HAART) which restores immunity. However, microsporidian parasites are not eliminated in all patients and relapses could be observed. Otherwise, healthy persons, especially travelers returning from tropical areas and
immunosuppressed patients undergoing organ-transplantation are still at threat of such opportunistic infection.
Stool examination for the diagnosis of microsporidial infections currently uses special staining techniques (Weber, Uvitex 2B), which requires a high level of expertise in order to be reliable, and PCR, which is expensive and time consuming. We supply here two monoclonal antibodies specific for
E. bieneusi spores (1.3 x 0.7
mm) and E. intestinalis
spores (1.7 x 1.0 - 1.1 mm)
developed to screen stools by indirect immunofluorescence assay. This diagnostic method showed to be reliable, rapid and specific for the diagnosis and epidemiological survey of microsporidiosis.
Species identification has been possible only using transmission electron microscopy or PCR. The two monoclonal antibodies enable identification of both species. Differentiation between the two intestinal microsporidia is required for an adequate therapy management.
E. intestinalis infections are treated with albendazole, while fumagillin has been shown to be effective for eradicating
E. bieneusi. Thus, species identification is important for defining the appropriate treatment.
| Test
kit (2x50 assays): 2 x 0.5 ml anti-Enterocytozoon bieneusi monoclonal antibody, ready to use 2 x 0.5 ml anti-Encephalitozoon intestinalis monoclonal antibody, ready to use 1 x 2.0 ml fluorescent (488nm) goat anti-mouse IgG conjugate, ready to use Instructions for use (staining procedure) |
|
Sensitivities and specificities:
A sensitivity close to 100 % and a specificity
better than those of specific staining methods (Weber and Uvitex 2B) were
observed using the monoclonal antibodies and IFA.
References:
Simple species diagnosis of human intestinal microsporidia by an immunofluorescence test using specific monoclonal antibodies : an evaluation study in two hospitals in France. M. Thellier, I. Accoceberry , I. Desportes, S. Biligui, E. Bart-Delabesse, C. Ripert, M. Danis, A. Datry. . First United Workshop on Microsporidia from Invertebrate and Vertebreate Hosts (NATO). July 12-15,2004. Ceské Budéjovice.
Abstract
European directive 98/79/EC registration
Material Safety Data Sheet: Eng - Fra - Deu
BORDIER
AFFINITY PRODUCTS S.A.
![]() Chemin
de Chatanerie 2, 1023 Crissier, Switzerland.
Chemin
de Chatanerie 2, 1023 Crissier, Switzerland.
![]() +41
21 633 31 67
+41
21 633 31 67 ![]() cb@bordier.ch
cb@bordier.ch ![]() www.bordier.ch
www.bordier.ch